The slightest error or misuse of equipment could lead to an expensive risk, delay manufacturing of a product and minimize the amount of sales. It is necessary to have all items involved in the manufacturing process go through a qualification test. Equipment qualification is extremely critical. Qualification needs to be documented and recorded for both equipment and products in the pharmaceutical industry.
A series of inspections, tests and assessments are performed on each piece of equipment to ensure quality. Before they are used for manufacturing products that are distributed to patients.
Below is a diagram provided from Pharmaceutical Online of how information and data is tracked for qualifications.
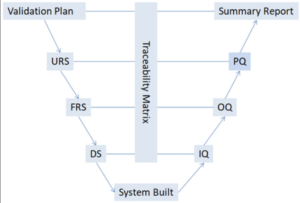
Many documents are required throughout the inspection/ qualification process. These documents are organized into three groups; Specification, Protocol and Report. Once equipment qualification is completed and all documentation is finalized, a report is created to prove quality and allowance to manufacture drug products. Constant reviews of the equipment will need to be tracked and traced to ensure product quality.
Tracking and storing data on a paper-based system is outdated and difficult to trace. InQuest Science developed the Identifier Software that tracks and trends all data, provides guidance with qualifications that is needed for each product and able to store data in a cloud-based server. Our software helps your company’s workflow when qualifying all products in the manufacturing department of a company.
To learn more about equipment qualifications read the full article published by Pharmaceutical Online.

