 Ongoing advancement in technology, leads to increased development of parenteral drugs—one of the fastest growing choice for drug delivery in patients. Typically, manufacturing companies fill vials of products and ship them to physicians, pharmacies, etc. with an unfilled syringe; therefore, creating more work for all parties involved. Introducing prefilled syringes could cut manufacturing costs and product costs for the pharmaceutical companies; as well as benefitting patients and physicians.
Ongoing advancement in technology, leads to increased development of parenteral drugs—one of the fastest growing choice for drug delivery in patients. Typically, manufacturing companies fill vials of products and ship them to physicians, pharmacies, etc. with an unfilled syringe; therefore, creating more work for all parties involved. Introducing prefilled syringes could cut manufacturing costs and product costs for the pharmaceutical companies; as well as benefitting patients and physicians.
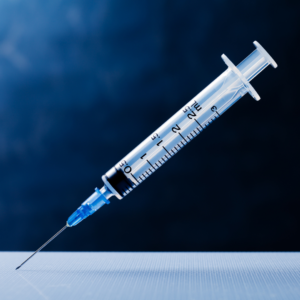
Making the transition from Vials to Prefilled Syringes helps others based on their needs or requirements. The reason for the switch could be from their change in the route of administration, increase the dose and the preference to administer an injection, minimizes waste, preference to administer lower dosages and many other reasons.
Typically, vials could be overfilled by up to 30% to ensure that each syringe will obtain a full dosage—proving product waste. With prefilled syringes, product is already filled and administered by need.
Prefilled syringes will help with inspection process—shortening the amount of product that need to be inspected prior to distribution will help save time and money for manufacturing companies.
InQuest Sciences’ new Identifier Software could help manufacturers track at trace all data involved in the transition of creating prefilled syringes. Our software allows you to maintain a better workflow, following all requirements in CFR part 11 and USP <790> and <1790>. When transitioning to prefilled syringes to support a better workflow, think of InQuest Sciences’ Identifier Software to help cement the workflow in your manufacturing process.

