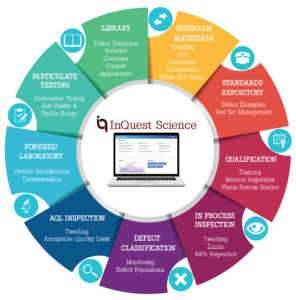
Identifier Life-Cycle Modules
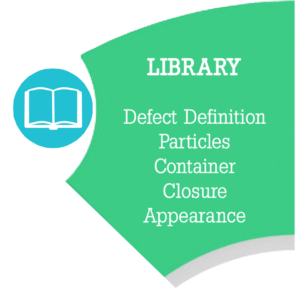
Library Module – Defines all Defects. Digitally provides a master description, images and criticality category (Critical, Major or Minor) for all defects intrinsic to incoming product contact materials or finished products. Particulate matter, product appearance and physical (container/closure) defect parameters are documented. The Library supports the defect training and process knowledge.
Expected by FDA and recommended in USP<1790> Sections:
- 1.3 Use of libraries containing visual standards (e.g., photographs and/or drawings) and characterized defect samples.
- 4.1, Particle Definitions Extrinsic, Intrinsic, or Inherent Particles
- 7.7 Training and Qualification of Human Inspectors, initially train the inspectors with defect photographic or video library and clear, written descriptions.
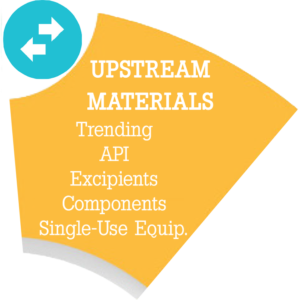 Upstream Materials Module – Upstream Defect Control. Foreign particulate matter and critical container or closure defects exist in all components, raw materials, API, single-use equipment and supplies that contact the product. The product control lifecycle can only be effective if these upstream contaminants or critical defect attributes are evaluated prior to acceptance for use in the final manufacturing of the product.
Upstream Materials Module – Upstream Defect Control. Foreign particulate matter and critical container or closure defects exist in all components, raw materials, API, single-use equipment and supplies that contact the product. The product control lifecycle can only be effective if these upstream contaminants or critical defect attributes are evaluated prior to acceptance for use in the final manufacturing of the product.
Expected by FDA and recommended in USP<1790> Sections:
- 4.1, 4.2 & 4.3 Recommendations for Prevention and Control of Specific Components and Processes.
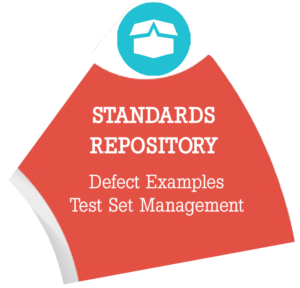 Defect Standards Repository Module – Stores all Defect Examples. Provides an inventory of actual units that represent specific defects intrinsic to incoming materials or finished products. The defects and blank units are assigned to specific “Test Sets” used during detection capability or Knapp studies of human inspectors or automated vision systems. Defect test sets are required for training inspectors or vision systems and demonstrating the sensitivity of the inspection method’s defect detection capabilities.
Defect Standards Repository Module – Stores all Defect Examples. Provides an inventory of actual units that represent specific defects intrinsic to incoming materials or finished products. The defects and blank units are assigned to specific “Test Sets” used during detection capability or Knapp studies of human inspectors or automated vision systems. Defect test sets are required for training inspectors or vision systems and demonstrating the sensitivity of the inspection method’s defect detection capabilities.
Expected by FDA and recommended in USP<1790>:
- 7.1 Standards
- 7.2 Preparing Defect Standards
- 7.3 Particle Types
- 7.4 Rejection Probability Determination
- 7.5 Test Sets
- 7.6 Types of Test Sets
 Qualification Module - Training, Inspector and Vision System Studies. The test set standards in the Repository are “calibrated” for probability of detection and used to train qualify manual and automated visual inspection particulate or defect detection methods and inspectors. Blinded manual baseline and Knapp detection studies are documented and analyzed electronically to maintain un-biased data integrity.
Qualification Module - Training, Inspector and Vision System Studies. The test set standards in the Repository are “calibrated” for probability of detection and used to train qualify manual and automated visual inspection particulate or defect detection methods and inspectors. Blinded manual baseline and Knapp detection studies are documented and analyzed electronically to maintain un-biased data integrity.
Expected by FDA and recommended in USP<1790> Sections:
- 6.1 Manual Visual Inspection
- 6.2 Semi-Automated Visual Inspection
- 6.3 Automated Visual Inspection
- 7.6 Types of Test Sets
- 7.7 Training and Qualification of Human Inspectors
- 7.8 Inspector Qualification Requirements
- 7.9 Requalification
 In Process Inspection Module – All Finished Product Units. The inspection of each individual sterile or injectable unit is required by USP<790>, to assure that all known detectable defects are securely removed from the batch of finished product prior to release. This is a requirement for sterile dosage forms.
In Process Inspection Module – All Finished Product Units. The inspection of each individual sterile or injectable unit is required by USP<790>, to assure that all known detectable defects are securely removed from the batch of finished product prior to release. This is a requirement for sterile dosage forms.
Expected by FDA and recommended in USP<1790> Sections:
- 3.1 100% Inspection of Each Unit
- 4.4 Trending and Limits Evaluation
- Facilitates collation of data for Annual Product Reviews
 Defect Classification Module - Trends and Tracks Defect Populations. Particulate matter or other physical defects from rejected product must be classified and grouped in order to determine typical control levels, their origin and possible remediation activities.
Defect Classification Module - Trends and Tracks Defect Populations. Particulate matter or other physical defects from rejected product must be classified and grouped in order to determine typical control levels, their origin and possible remediation activities.
Expected by FDA and recommended in USP<1790> Sections:
- 5.1 Defect Classification
- Defects are commonly grouped into classifications based on patient and compliance risk. The most common systems group defects into particle types and physical defects of critical, major, and minor significance.
- Interactive with Forensic Particle ID Module
 AQL Inspection Module – Acceptable Quality Limit Inspection. The statistically based Defect Attributes Inspection is performed on the good fraction of product after the primary (100%) inspection. It is a final verification by the Quality Unit of the effectiveness of the primary 100% inspection to remove the visibly detectable defects from each batch. AQL inspection is required by USP<790> to determine if a batch is “essentially free” of foreign particles before release of the batch.
AQL Inspection Module – Acceptable Quality Limit Inspection. The statistically based Defect Attributes Inspection is performed on the good fraction of product after the primary (100%) inspection. It is a final verification by the Quality Unit of the effectiveness of the primary 100% inspection to remove the visibly detectable defects from each batch. AQL inspection is required by USP<790> to determine if a batch is “essentially free” of foreign particles before release of the batch.
Expected by FDA and recommended in USP<1790> Sections:
- 3.2 Acceptable Quality Limits (AQL) Inspection
- 4.4 Trending and Limits Evaluation
- Interactive with Forensic Particle ID Module
- Facilitates collation of data for Annual Product Reviews
 Forensic Lab Module - Particle Identification. Particulate matter identification is an essential tool in discovering the root cause of why product units are rejected during the inspection or testing process. Particle ID is supported at Level 1 (In-situ), Level 2 (Microscopic) and Level 3 (Spectroscopic) analysis. Knowing the process step and likely source of the contamination is the best way to eliminate the problem and facilitates CAPA activities to prevent reoccurrence.
Forensic Lab Module - Particle Identification. Particulate matter identification is an essential tool in discovering the root cause of why product units are rejected during the inspection or testing process. Particle ID is supported at Level 1 (In-situ), Level 2 (Microscopic) and Level 3 (Spectroscopic) analysis. Knowing the process step and likely source of the contamination is the best way to eliminate the problem and facilitates CAPA activities to prevent reoccurrence.
Expected by FDA and recommended in USP<1790> Sections:
- 3.1 Rejects from 100% Inspection
- 3.2 Rejects from Acceptable Quality Limits (AQL) Inspection
- 4.1 Particle Definitions Extrinsic, Intrinsic, or Inherent Particles
- 5.1 Defect Classification
- Supports all other Modules with Particulate Matter Characterization Documentation or ID Data
- Internal Characterization or External Laboratory Data
 Particle Testing Module – Destructive Sub-visible and Visible Range Testing. All sterile products must meet the limits for sub-visible particulates established in USP<771>, <787>, <788> & <789>. In addition, supplemental testing for Difficult to Inspect Products (DIP) must be conducted to assure the foreign particle burden in the visible range (>100µm) is known and meets the “Essentially Free” from visible particles required by USP<790>.
Particle Testing Module – Destructive Sub-visible and Visible Range Testing. All sterile products must meet the limits for sub-visible particulates established in USP<771>, <787>, <788> & <789>. In addition, supplemental testing for Difficult to Inspect Products (DIP) must be conducted to assure the foreign particle burden in the visible range (>100µm) is known and meets the “Essentially Free” from visible particles required by USP<790>.
Expected by FDA and recommended in USP<1790> Sections:
- 5.2 Unique Product and Container Considerations – Destructive Testing
- 5.3 Alternate Inspection Strategies for Supplemental Testing
- 4.4 Trending and Limits Evaluation
- Interactive with Forensic Particle ID Module
- Facilitates collation of data for Annual Product Reviews